Density of Nacl Solution
A 15L solution is composed of 025g NaCl dissolved in water. Since the solutions are mostly water the solutions are assumed to have a density of 10 gmL and a specific heat of 418 JgC.

Variation Of Nacl Aqueous Solutions Density With Temperature And Nacl Download Table
M nV where n is the number of moles and V is the volume in litres.
. Calculating the molar enthalpy of neutralisation using the data from the experiment. Lets show on the example how to calculate molality. 680 g NaCl x 1 mole NaCl 0116 mole NaCl 5845 g NaCl 2750 mL H 2 O x 1 g H 2 O x 1 mole H 2 O 1528 mol H 2 O.
Solve Study Textbooks Guides. Q-Although phenoxide ion has more number of resonating structures than carboxylate ion carboxylic acid is a stronger acid than phenol. The density of ethylene glycol antifreeze HOCH 2 CH 2 OH is 109 gmL.
99 pure sodium chloride NaCl was used to minimize the possibility of interference from other ions. When salt sodium chloride or NaCl dissolves in water there is a significant increase in mass of the solution due to the relatively higher molecular mass of the dissolved ions Na 22 gmol and Cl 355gmol when compared to water or H 2 O 20 gmol. We can rearrange this equation to get the number of moles.
You multiply the molarity by the volume in litres. The official symbol for molarity is c concentration but many people use the old symbol M. Calculate the molality of the solution.
2 How many grams of magnesium cyanide are needed to make 275 mL of a 0075. Find the mass percent volume percent and massvolume percent of the solute. When the chloride solution penetrates the concrete and.
I density of each dilute aqueous solution is the same as water 1 g mL-1 at 25C so the mass of solution in grams volume of solution in mL ii heat capacity of each solution is the same as for water C g 418 JC-1 g-1. 3 Molar solution means there are 3 moles of NaCl salt in 1 Liter. With molar masses of 2299 and 3545 gmol respectively 100 g of NaCl contains 3934 g Na and 6066 g Cl.
Interestingly the dissolved salt does not increase the volume of the water by the volume of the added salt and this is due to the. Mass of 1 litre of solution 125 gmsml 1000 ml 1 250 gms V volume of water added to make the solution or volume of solvent Mass of solute Mass of solvent Mass of solution 17532 gms mass of solvent 1 250 gm mass of solvent 1250-17532 10748 So 1 07468 gms of water is mixed with 3 moles of NaCl to make the 3 M solution. Add 5844 grams of salt for a 1M solution and add 11688 grams and so on to make a 2M solution.
36g of NaCl s will dissolve in 100 mL of water at 25C. A saturated solution 23 wv freezes at -205C 5F. Sodium chloride ˌ s oʊ d i ə m ˈ k l ɔːr aɪ d commonly known as salt although sea salt also contains other chemical salts is an ionic compound with the chemical formula NaCl representing a 11 ratio of sodium and chloride ions.
D - mass density of the solution. NaCl and KCl were used as molten salts and the cathode material synthesized with NaCl exhibited more isotropic morphologies than the material synthesized with KCl. Add 585 g of NaCl to water to make 1L of solution.
Join Login Class 12 Chemistry. Sodium chloride is the salt most. Molarity is the number of moles of a substance in one litre of solution.
Is NaCl crystalline or amorphous solid. NaCl is unusual in that its solubility does not increase appreciably with temperature since at 100C the solubility is 384 mgml. With the solution shown in the picture below find the mole percent of substance C.
Now you have 250 ml of water which is about 250 g of water assuming a density of 1 gml but you also have 3 grams of solute so the total mass of the solution is closer to. Visit BYJUS to understand the properties. N M V.
Click hereto get an answer to your question The density of a 3M solution of NaCl is 125gml. If NaCl is doped with 10-3 mol of SrCl 2. Freezing Point Depression Problem.
In an ideal solution freezing point depression only depends on solute concentration. How to calculate molality with this molality calculator. HIT THE BACK BUTTON ON YOUR.
An experimental study on the steel corrosion behavior in NaCl-immersed concrete under. To prepare a 1M solution of sodium chloride Q. The NaCl Molecular Weight Sodium Chloride is 5844 gmol.
Moles of NaCl 0542 mol kg water density x volume kg water 0994 gmL x 220 mL x 1 kg1000 g kg water 0219 kg m NaCl moles of NaClkg water. In a solution there is 1110 mL 110605 g solvent and 524 mL 60508 g solute present in a solution. This solution will be a saturated aqueous solution of sodium chloride but there will be no visible solid NaCl in the vessel.
Determine the mole fraction of NaCl in a solution in which 010 moles of the salt is dissolved in 100 grams. Synthesized LiNi 08 Co 01 Mn 01 O 2 cathode material using NaCl and KCl using the molten-salt method and the particles using NaCl exhibited 101 and 003 facets with almost perfect. Express this as moles per kilogram solution.
Of the sodium chloride and of the water in the solution. Density of sodium chloride. Boiling Point of sodium chloride.
Maximum solubility of NaCl in water at 25C is 357 mgml 263. River sand was used as the fine aggregate with a bulk density of 1680 k g m 3. If 40 g of NaCl s is added to 100 mL of water at 25C only 36g of NaCl s will dissolve 4 g of the NaCl s will not dissolve.
Q solution m c T where m is the total mass of the resultant solution and c is the specific heat capacity of the resultant solution. The density of a saturated solution at 25C is 1202 gml. If the density of solution is 12 g mL-1 then what shall be the molarity of the solution.
Divide 12 mol by 15 kg and youll find out that the molality of the NaCl solution is. The heat gained by the resultant solution can be calculated using. How many grams of solution will contain 305 g of NaCl if the solution has a concentration of 83.

Variation Of Nacl Aqueous Solutions Density With Temperature And Nacl Download Table

Variation Of Nacl Aqueous Solutions Density With Temperature And Nacl Download Table

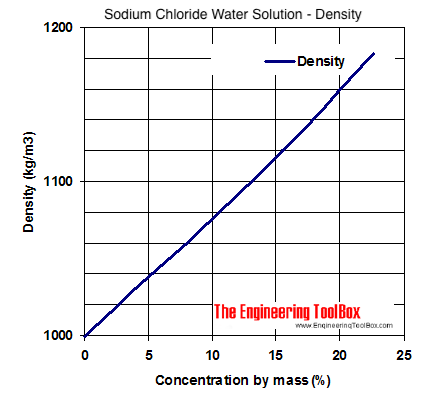
Sodium Chloride Water Solutions
0 Response to "Density of Nacl Solution"
Post a Comment